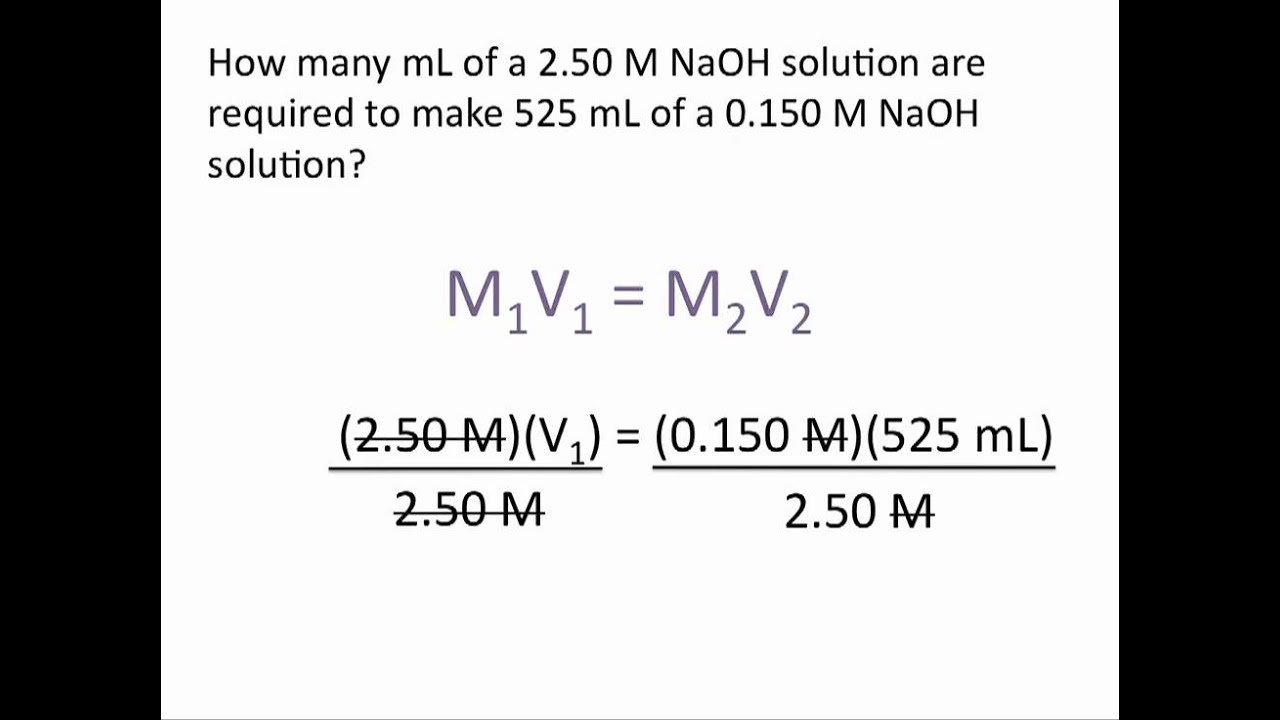
Dilution Buffer Equation . if you look at the buffer formula, ph = pka + lg [salt]/ [acid], dilution does not affect the [salt]/ [acid] ratio. how do buffer solutions work? A mixture of a weak acid and. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. an equation that could calculate the ph value of a given buffer solution was first derived by the american chemist lawrence joseph. It is able to neutralize small.
from www.youtube.com
an equation that could calculate the ph value of a given buffer solution was first derived by the american chemist lawrence joseph. A mixture of a weak acid and. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small. if you look at the buffer formula, ph = pka + lg [salt]/ [acid], dilution does not affect the [salt]/ [acid] ratio. how do buffer solutions work?
Dilution Problems Chemistry Tutorial YouTube
Dilution Buffer Equation an equation that could calculate the ph value of a given buffer solution was first derived by the american chemist lawrence joseph. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. an equation that could calculate the ph value of a given buffer solution was first derived by the american chemist lawrence joseph. A mixture of a weak acid and. It is able to neutralize small. how do buffer solutions work? if you look at the buffer formula, ph = pka + lg [salt]/ [acid], dilution does not affect the [salt]/ [acid] ratio.
From sciencequery.com
What is serial dilution method? And how to calculate? Science Query Dilution Buffer Equation how do buffer solutions work? It is able to neutralize small. an equation that could calculate the ph value of a given buffer solution was first derived by the american chemist lawrence joseph. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. if you look at. Dilution Buffer Equation.
From www.youtube.com
Dilution formula and how to use it to solve problems. 3 examples solved Dilution Buffer Equation an equation that could calculate the ph value of a given buffer solution was first derived by the american chemist lawrence joseph. A mixture of a weak acid and. if you look at the buffer formula, ph = pka + lg [salt]/ [acid], dilution does not affect the [salt]/ [acid] ratio. a buffer is a solution that. Dilution Buffer Equation.
From www.ck12.org
Buffer Solutions Overview ( Video ) Chemistry CK12 Foundation Dilution Buffer Equation A mixture of a weak acid and. how do buffer solutions work? if you look at the buffer formula, ph = pka + lg [salt]/ [acid], dilution does not affect the [salt]/ [acid] ratio. It is able to neutralize small. an equation that could calculate the ph value of a given buffer solution was first derived by. Dilution Buffer Equation.
From www.numerade.com
SOLVEDUsing the equation below, calculate the buffer capacity (BC) of Dilution Buffer Equation It is able to neutralize small. A mixture of a weak acid and. if you look at the buffer formula, ph = pka + lg [salt]/ [acid], dilution does not affect the [salt]/ [acid] ratio. how do buffer solutions work? an equation that could calculate the ph value of a given buffer solution was first derived by. Dilution Buffer Equation.
From chemistry.stackexchange.com
acid base Calculation of pH in diluted buffer. Chemistry Stack Exchange Dilution Buffer Equation an equation that could calculate the ph value of a given buffer solution was first derived by the american chemist lawrence joseph. It is able to neutralize small. how do buffer solutions work? if you look at the buffer formula, ph = pka + lg [salt]/ [acid], dilution does not affect the [salt]/ [acid] ratio. a. Dilution Buffer Equation.
From exoubqlmz.blob.core.windows.net
Dilution Equation With at Sharon Firestone blog Dilution Buffer Equation a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A mixture of a weak acid and. It is able to neutralize small. an equation that could calculate the ph value of a given buffer solution was first derived by the american chemist lawrence joseph. if you look. Dilution Buffer Equation.
From www.chegg.com
Solved EXAMPLE 5 DILUTIONS If I wanted to make 100 mL of a Dilution Buffer Equation a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. how do buffer solutions work? It is able to neutralize small. an equation that could calculate the ph value of a given buffer solution was first derived by the american chemist lawrence joseph. A mixture of a weak. Dilution Buffer Equation.
From www.cytivalifesciences.com
Inline dilution for chromatography buffer preparation Cytiva Dilution Buffer Equation how do buffer solutions work? an equation that could calculate the ph value of a given buffer solution was first derived by the american chemist lawrence joseph. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. if you look at the buffer formula, ph = pka. Dilution Buffer Equation.
From www.youtube.com
Dilution calculations Dilution problems Stock dilutions Biology and Dilution Buffer Equation A mixture of a weak acid and. an equation that could calculate the ph value of a given buffer solution was first derived by the american chemist lawrence joseph. if you look at the buffer formula, ph = pka + lg [salt]/ [acid], dilution does not affect the [salt]/ [acid] ratio. a buffer is a solution that. Dilution Buffer Equation.
From www.slideserve.com
PPT Chapter 16 Aqueous Ionic Equilibrium PowerPoint Presentation Dilution Buffer Equation how do buffer solutions work? a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small. if you look at the buffer formula, ph = pka + lg [salt]/ [acid], dilution does not affect the [salt]/ [acid] ratio. A mixture of a weak. Dilution Buffer Equation.
From www.youtube.com
Chem143 Dilution Equation YouTube Dilution Buffer Equation an equation that could calculate the ph value of a given buffer solution was first derived by the american chemist lawrence joseph. A mixture of a weak acid and. how do buffer solutions work? a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. if you look. Dilution Buffer Equation.
From www.slideserve.com
PPT Dilution Calculations PowerPoint Presentation, free download ID Dilution Buffer Equation It is able to neutralize small. if you look at the buffer formula, ph = pka + lg [salt]/ [acid], dilution does not affect the [salt]/ [acid] ratio. A mixture of a weak acid and. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. how do buffer. Dilution Buffer Equation.
From www.youtube.com
Dilution in biology lab How to use the equation C1V1 = C2V2 YouTube Dilution Buffer Equation It is able to neutralize small. A mixture of a weak acid and. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. if you look at the buffer formula, ph = pka + lg [salt]/ [acid], dilution does not affect the [salt]/ [acid] ratio. an equation that. Dilution Buffer Equation.
From www.youtube.com
CHEMISTRY 101 Solution Dilutions YouTube Dilution Buffer Equation a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. an equation that could calculate the ph value of a given buffer solution was first derived by the american chemist lawrence joseph. It is able to neutralize small. A mixture of a weak acid and. if you look. Dilution Buffer Equation.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free Dilution Buffer Equation A mixture of a weak acid and. It is able to neutralize small. how do buffer solutions work? an equation that could calculate the ph value of a given buffer solution was first derived by the american chemist lawrence joseph. a buffer is a solution that can resist ph change upon the addition of an acidic or. Dilution Buffer Equation.
From dxocmttnm.blob.core.windows.net
Dilution Equation Formulas at Kathleen Milford blog Dilution Buffer Equation an equation that could calculate the ph value of a given buffer solution was first derived by the american chemist lawrence joseph. It is able to neutralize small. A mixture of a weak acid and. if you look at the buffer formula, ph = pka + lg [salt]/ [acid], dilution does not affect the [salt]/ [acid] ratio. . Dilution Buffer Equation.
From www.chegg.com
Solved EXAMPLE A Dilute Buffer Prepared from a Moderately Dilution Buffer Equation how do buffer solutions work? It is able to neutralize small. if you look at the buffer formula, ph = pka + lg [salt]/ [acid], dilution does not affect the [salt]/ [acid] ratio. A mixture of a weak acid and. an equation that could calculate the ph value of a given buffer solution was first derived by. Dilution Buffer Equation.
From www.youtube.com
Chem 11 Dilution Calculations_Solving for Final Volume after Dilution Dilution Buffer Equation if you look at the buffer formula, ph = pka + lg [salt]/ [acid], dilution does not affect the [salt]/ [acid] ratio. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A mixture of a weak acid and. an equation that could calculate the ph value of. Dilution Buffer Equation.